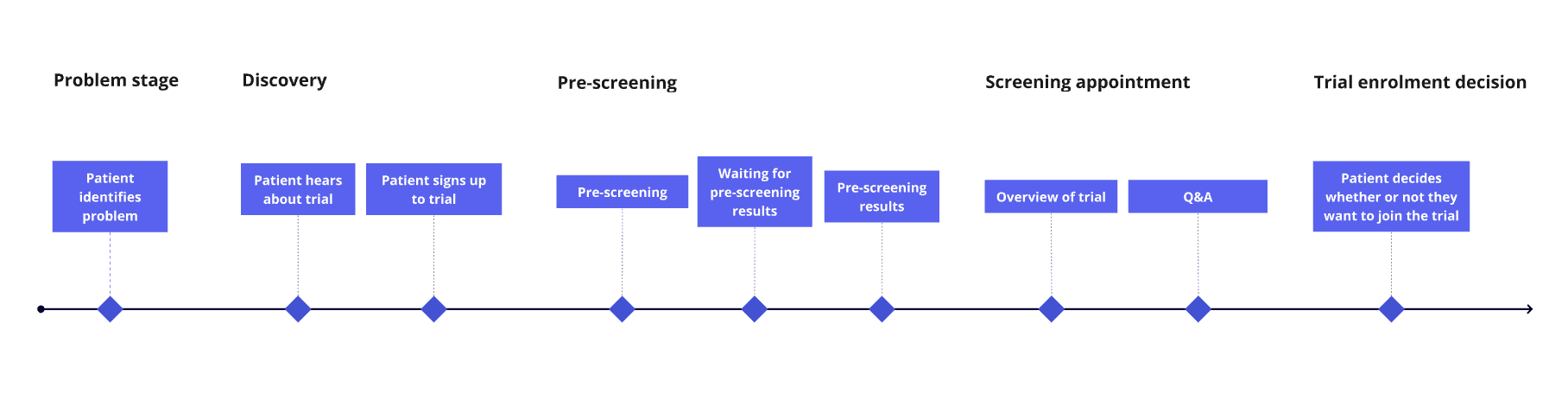
Recruitment process for a clinical trial participant
Quote from trial participant
Quote from trial participant
For this project, I took on responsibilities in product management, UX design, and UI design.
As a product manager I:
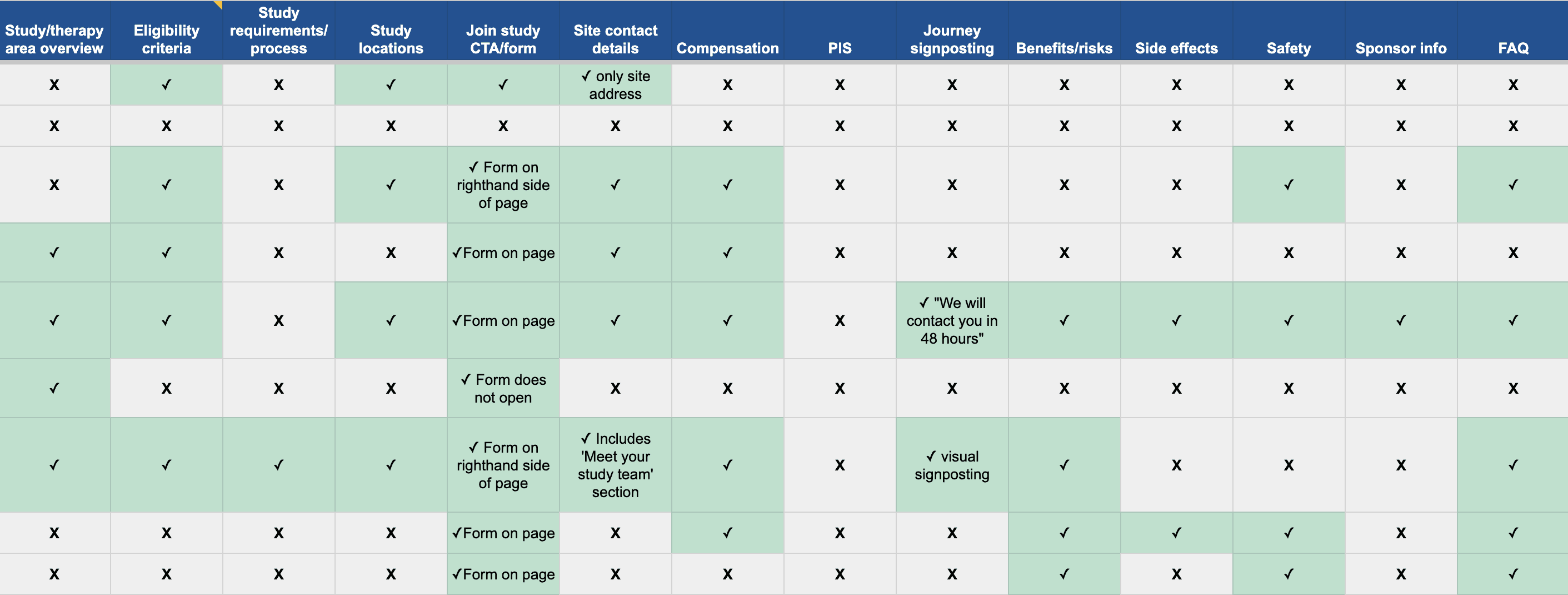
Conducted a competitive analysis of 12 site network landing pages to identify common design patterns, content blocks and opportunities to enhance the design and functionality of our landing page.
Communicated and collaborated with c-suite and engineering to get their perspective on what content blocks were needed, and also planned how this would fit in to our existing product.
Worked with our Senior Product Manager to scope the project.
Competitive analysis of site network landing pages
After scoping the project I designed wireframes for the landing page, focusing on highlighting key details, reassuring participants and conversion.
Highlighting key details
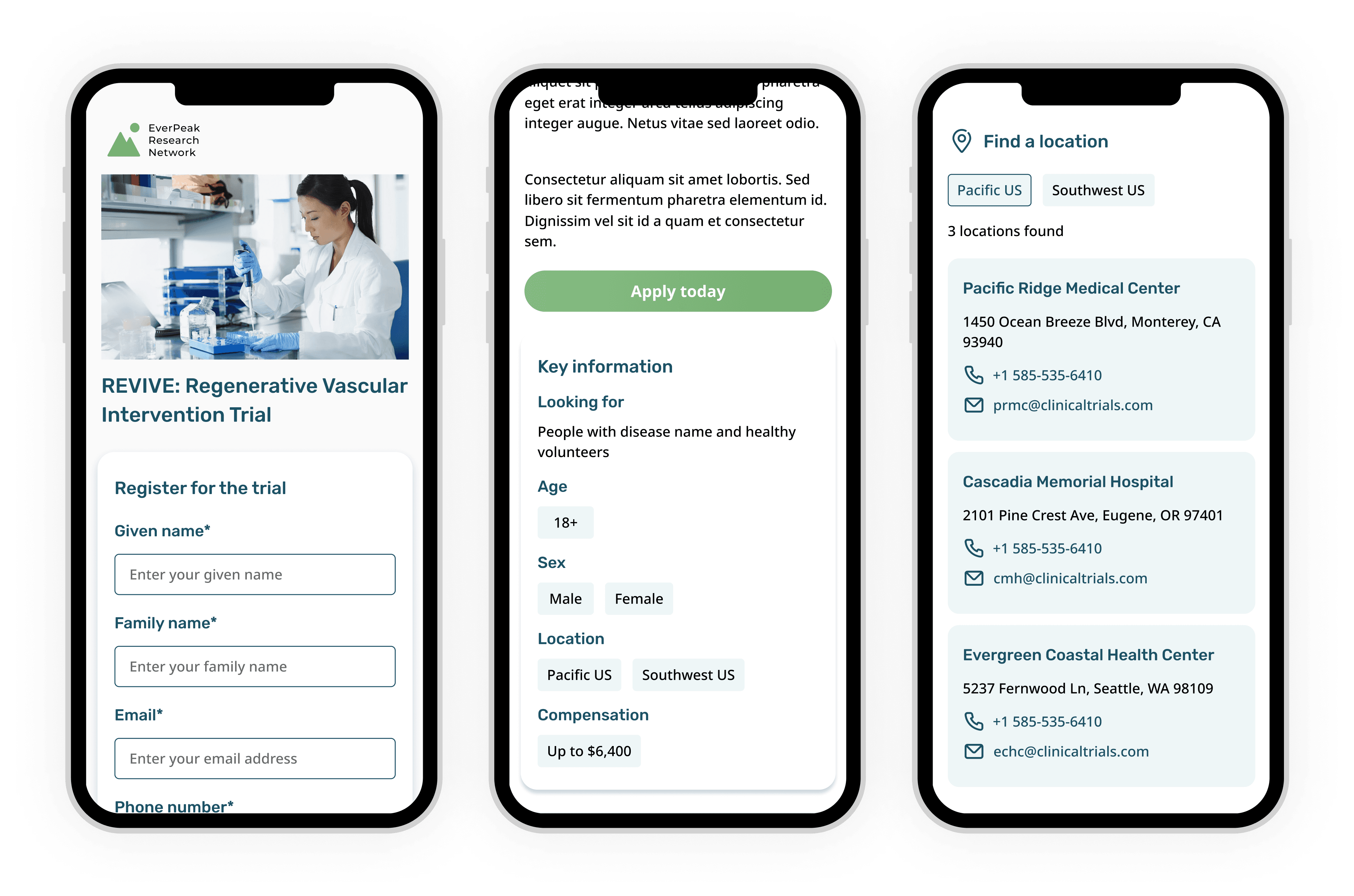
I designed components to showcase key study details, inclusion and exclusion criteria, enabling participants to quickly determine if the study aligns with their needs and eligibility
Reassuring participants
I designed a component to feature participant testimonials, addressing safety and trust concerns by showcasing patient experiences
I also wanted to make the study team feel more approachable, so sites can choose to share photos and contact details of their research team with participants
Conversion
CTAs are spread throughout the landing page to make it easier for participants to apply for the study
Desktop design
Mobile designs
To ensure the landing page could adapt to the brands of different site networks, we worked to identify which UI elements should be customised.
We agreed that font and icon colours, buttons, input fields, gradients, and backgrounds should be configurable to match each brand's colour palette.
However, to maintain readability and consistency across landing pages, we decided on sticking with Stitch’s brand fonts, using Rubik for headers and Noto Sans for body text.
Colour palette
Branded landing pages
Quote from Luke Snedaker (CEO of WCR)
“Stitch gives our participants a user interface that pulls clinical research into this decade; with beautiful design, useful information about their visit, and the ability to give feedback in a seamless platform.
The best part is that workload has been REDUCED on our coordinators and administrative team by adding this system to our tech stack.”